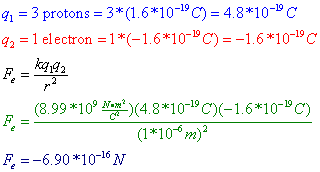Coulomb's Law
We know that like charges repel, and opposite charges attract. In order for charges to repel or attract, they apply a force upon each either. Similar to the manner in which the force of attraction between two masses is determined by the amount of mass and the distance between the masses, as described by Newton's Law of Universal Gravitation, the force of attraction or repulsion is determined by the amount of charge and the distance between the charges. The magnitude of the electrostatic force is described by Coulomb's Law.
Coulomb's Law states that the magnitude of the electrostatic force (Fe) between two objects is equal to a constant, k, multiplied by each of the two charges, q1 and q2, and divided by the square of the distance between the charges (r2). The constant k is known as the electrostatic constant, and is given as: ![]() .
.
Notice how similar this formula is to the formula for the gravitational force! Both Newton's Law of Universal Gravitation and Coulomb's Law follow the inverse-square relationship, a pattern that repeats many times over in physics. The further you get from the charges, the weaker the electrostatic force. If you were to double the distance from a charge, you would quarter the electrostatic force on a charge.

Formally, a positive value for the electrostatic force indicates that the force is a repelling force, while a negative value for the electrostatic force indicates that the force is an attractive force. Because force is a vector, you must assign a direction to it. To determine the direction of the force vector, once you have calculated its magnitude, use common sense to tell you the direction on each charged object. If the objects have opposite charges, they are being attracted, and if they have like charges, they must be repelling each other.
Question: Three protons are separated from a single electron by a distance of 1*10-6 m. Find the electrostatic force between them. Is this force attractive or repulsive?
Answer:
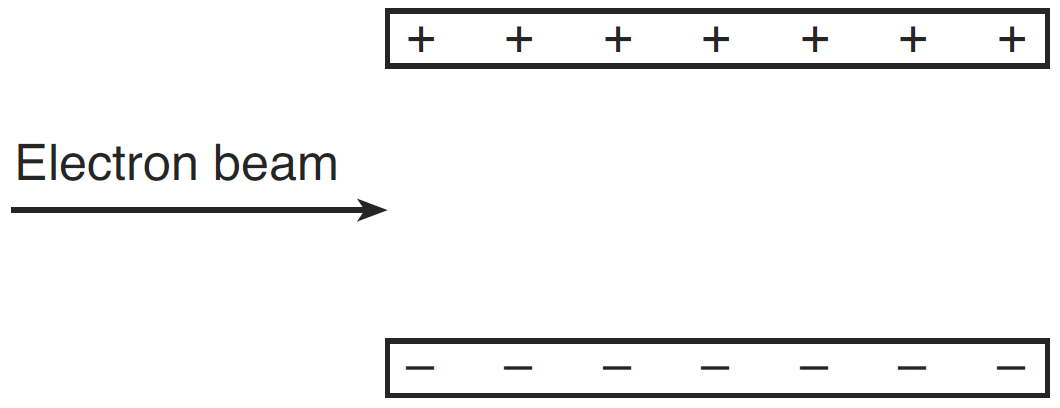
Question: A beam of electrons is directed into the electric field between two oppositely charged parallel plates, as shown in the diagram below.
The electrostatic force exerted on the electrons by the electric field is directed
- into the page
- out of the page
- toward the bottom of the page
- toward the top of the page
Answer: (4) toward the top of the page because the electron beam is negative, and will be attracted by the positively charged upper plate and repelled by the negatively charged lower plate.
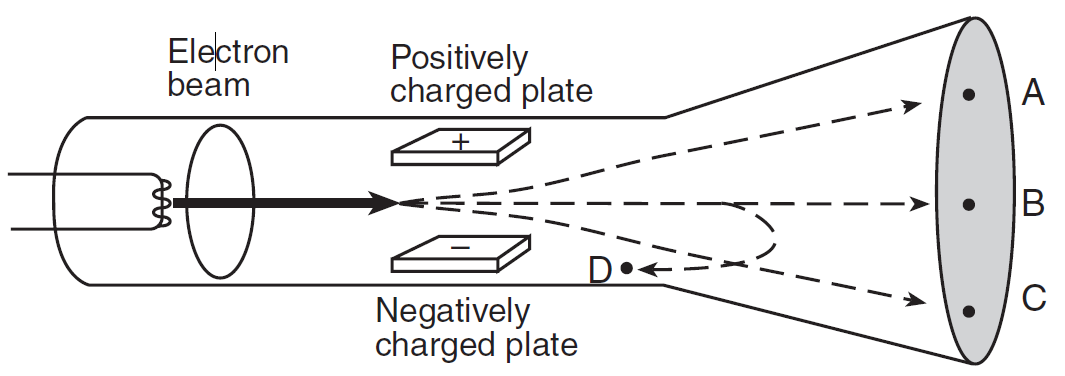
Question: The diagram below shows a beam of electrons fired through the region between two oppositely charged parallel plates in a cathode ray tube.
After passing between the charged plates, the electrons will most likely travel path
- A
- B
- C
- D
Answer: (1) A